
Fluorescence is a type of photoluminescence. Photoluminescence refers to the process of light re-emission after a material has absorbed photons. Unlike reflection and scattering, the wavelength of the emitted light is longer than the wavelength of the absorbed light. When a fluorescent or phosphorescent material is irradiated with high-energy ultraviolet (UV) light, it enters an excited state due to electronic transitions.
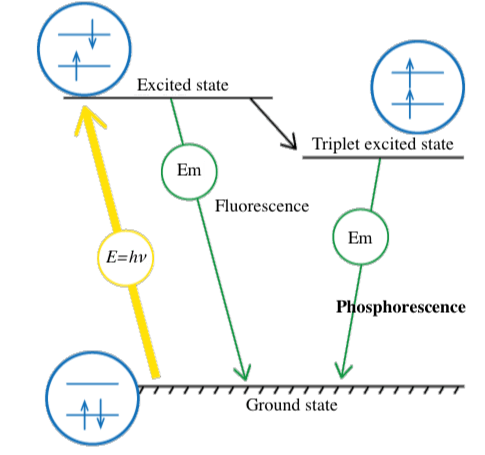
Luminescence occurs when electrons return from the excited state to the ground state. One example of a living organism that emits luminescence is Aquaria victoria (Figure 1). Luminescence is a generic term for light emission following absorption of optical energy.
The energy contained in a photon is expressed by E = hν (E: energy, h: Planck’s constant, ν: frequency). When light hits a substance, its energy is absorbed by electrons, and the electronic configuration changes from the stable ground state to a higher energy excited state.
Luminescence occurs when this energy is released in the form of light emission rather than molecular vibrations or heat generation. The electronic configuration then returns to the ground state (Figure 2). In many cases, part of the absorbed energy is emitted as thermal energy, so that the emitted light has a longer wavelength than the absorbed light (Stokes shift).
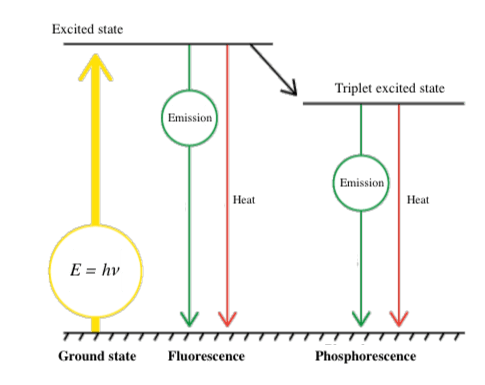
Light emission as a result of photo-excitation can occur partially in the form of fluorescence and partially as phosphorescence. Phosphorescence occurs when an intersystem crossing occurs, and electrons cannot return directly to the ground state, but instead make a transition to an intermediate state. They then relax slowly to the ground state, resulting in phosphorescence.
Fluorescence occurs on a timescale of nanoseconds, whereas phosphorescence can continue for much longer periods. When electronic transitions occur in a substance, electron pairs generally have opposite spins (Pauli’s exclusion principle). However, when an intersystem crossing occurs, the spins are the same. Since this is a forbidden state, relaxation to the ground state is slow.
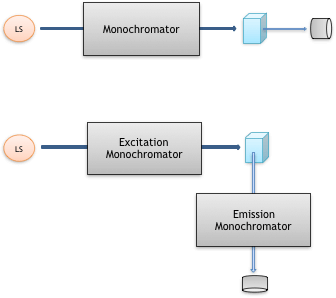
Figure 4 shows the typical setup of a UV/Vis spectrometer and a spectrofluorometer. Both are the same in that a sample is irradiated with monochromatic light from a light source after passing through a monochromator. The UV/Vis spectrometer detects light that has passed directly through the sample (absorption spectroscopy). In contrast, a spectrofluorometer detects fluorescent light emitted in the 90° direction and passed through an emission monochromator (emission spectroscopy).
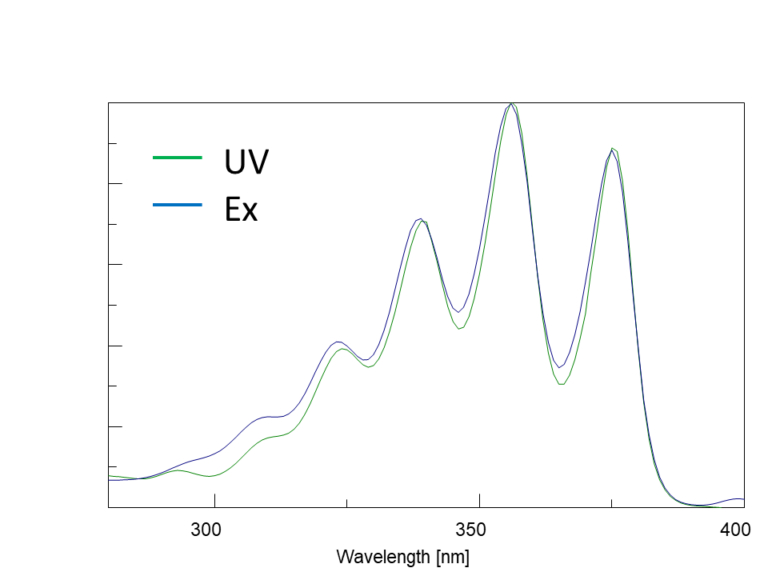
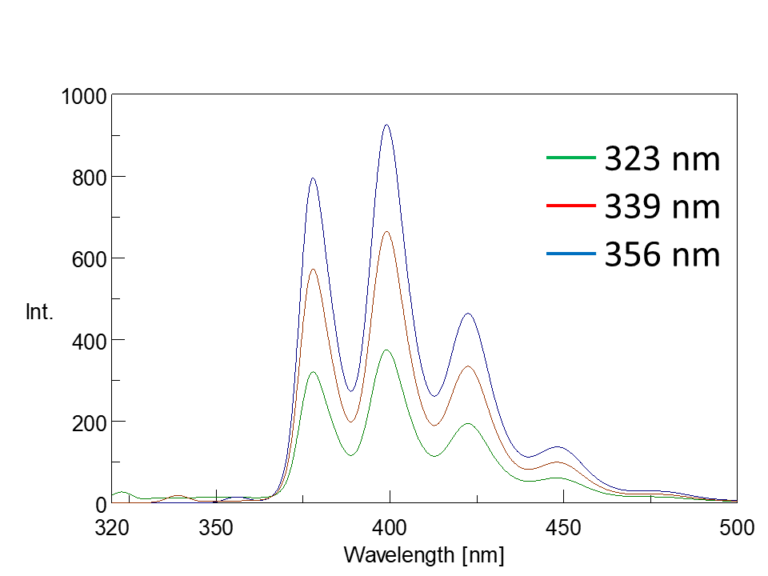
In the excitation spectrum, the emission wavelength (Em) is fixed, and the excitation wavelength (Ex) is scanned to measure the fluorescence intensity. The wavelength that gives the maximum fluorescence intensity is the maximum excitation wavelength. The shape of the excitation spectrum is similar to that of the absorption spectrum (Figure 5-1). The fluorescence spectrum is acquired by fixing the excitation wavelength (Ex) and scanning the emission wavelength (Em). Although the fluorescence intensity varies with excitation wavelength, the spectral profiles are similar (Figure 5-2).
©Jasco Europe S.R.L. (Socio Unico) Direzione e coordinamento ex. art. 2497 bis c.c. – P.I. 08609570158 | Privacy Policy | Cookie Policy | Manage consent | Realizzazione sito web: Alkimedia
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| CookieLawInfoConsent | 1 year | Records the default button state of the corresponding category & the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | This cookie is used by the website's WordPress theme. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
| Cookie | Duration | Description |
|---|---|---|
| Google Maps | Google Maps is a map visualization service managed by Google Inc. and is used to integrate such contents within its pages. |