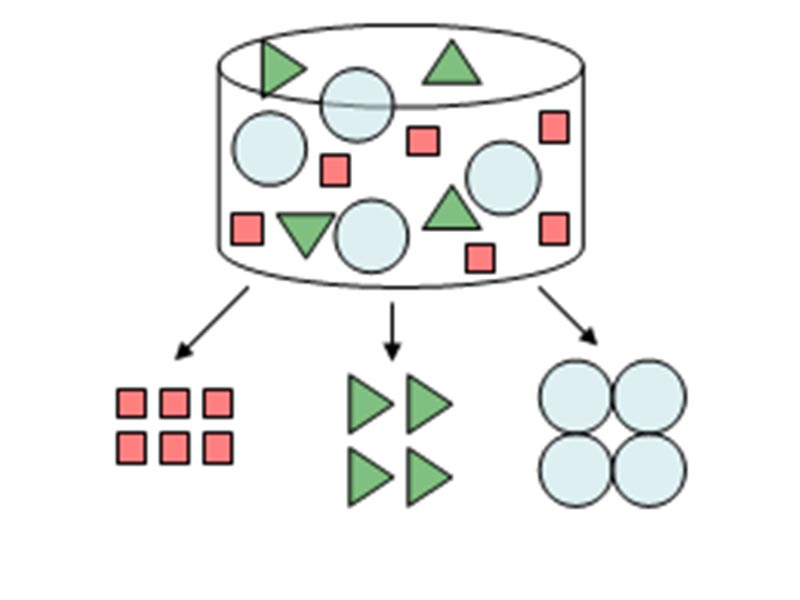
HPLC is an abbreviation for High Performance Liquid Chromatography. Chromatography refers to the measurement method, chromatogram refers to the measurement results, and chromatograph refers to the instrument. Chromatography separates components in a particular substance and performs qualitative and quantitative analyses. Qualitative analysis refers to “what kind of compound each component is”, and quantitative analysis refers to “how much of each component is present”
(Figure 1).
Methods for separating mixed compounds include filtration, distillation, and extraction. Chromatography was invented by the Italian/Russian botanist Mikhail Semenovich Tswett. In the early 1900s, Tswett packed calcium carbonate in a standing tube, placed pigments extracted from plants on top, and then flushed the tube with petroleum ether as a solvent (Figure 2). Individual pigments appeared as color bands as if the light was divided into seven colors by a prism in the tube.
For this reason, Tswett named this separation method chromatography using the Greek words chroma (color) and graph (record). High-performance liquid chromatography (HPLC) is a type of separation method referred to as column chromatography, and has been developed to enable separation and analysis in a short time using high pressure.
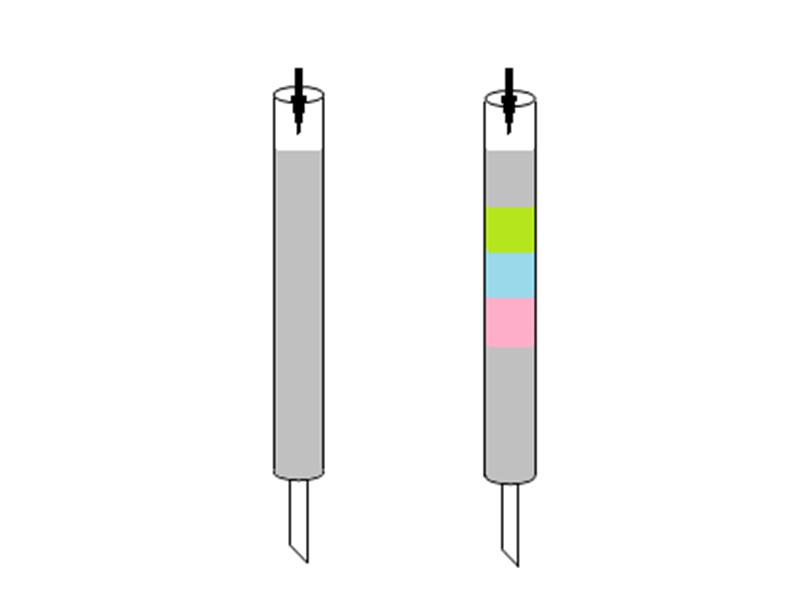
A mixture is placed in a stream of liquid (petroleum ether in Figure 3) called the mobile phase and moved through a solid medium (calcium carbonate powder in Figure 3) called the stationary phase. The components in the mixture move with the flow of the mobile phase, and interact with the stationary phase. The speed of movement depends on the strength of the interaction between each component and the stationary phase.
That is, components that interact strongly with the stationary phase move slowly, whereas components that interact weakly move quickly, so allowing the components to be separated.
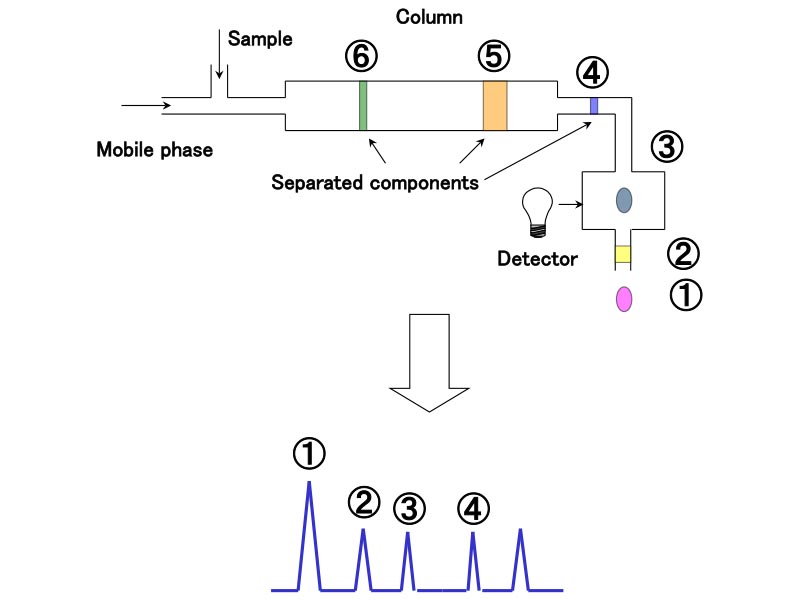
The separated components can be analyzed using different types of detectors. A UV detector, for example, can detect components based on UV absorption. The chromatogram is obtained by measuring the elution time on the X axis and the intensity of the UV signal on the Y axis. If the measurement conditions are the same, the elution time (peak position) for the standard sample whose components are known and that for the unknown sample can be compared to identify the components. In addition, since the absorption intensity is proportional to the concentration, a calibration curve can be prepared using a standard sample, and the component concentration can be determined by measuring the peak area or height.
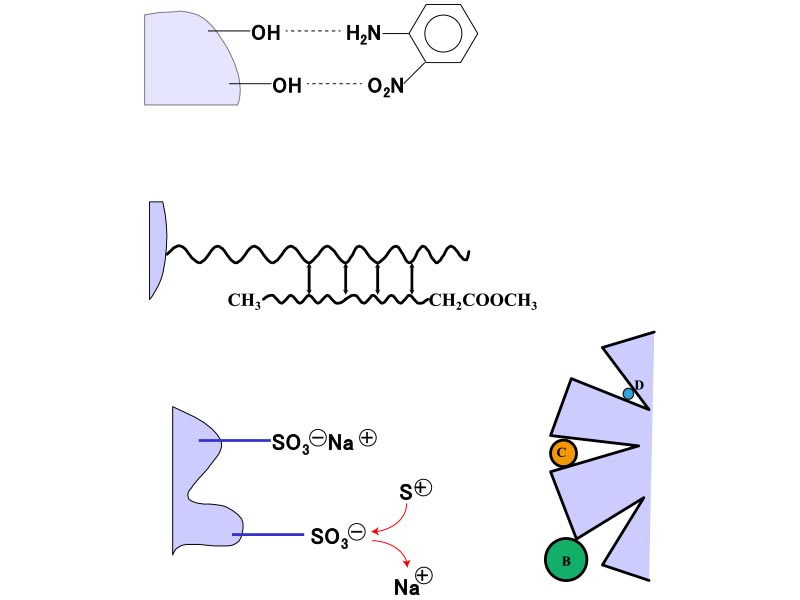
In HPLC, individual components are separated using a column, based on the difference in the degree of interaction between the sample components and the column. Components with a low degree of interaction with the column are eluted first. These interactions include adsorption, hydrophilic interactions, hydrophobic interactions, electro-affinity, penetration and exclusion (Figure 4).
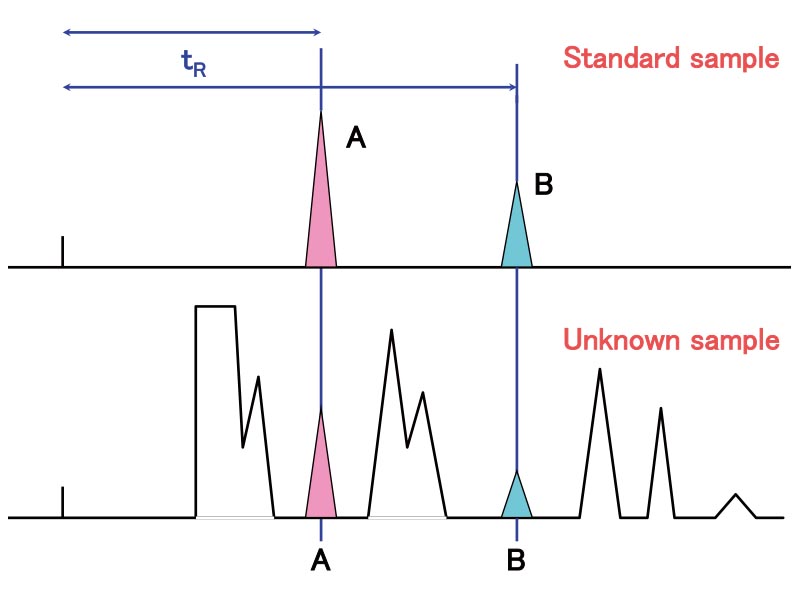
In most cases, identification of a sample component is performed by comparing its retention time with that in a standard sample. If a complex chromatogram with many peaks is obtained or if the retention time of the target component differs between the standard and the actual sample, the target component is identified by adding the standard sample to the unknown sample (Figure 5). Analyzing the HPLC-collected components by IR or mass spectroscopy enables reliable qualitative analysis.
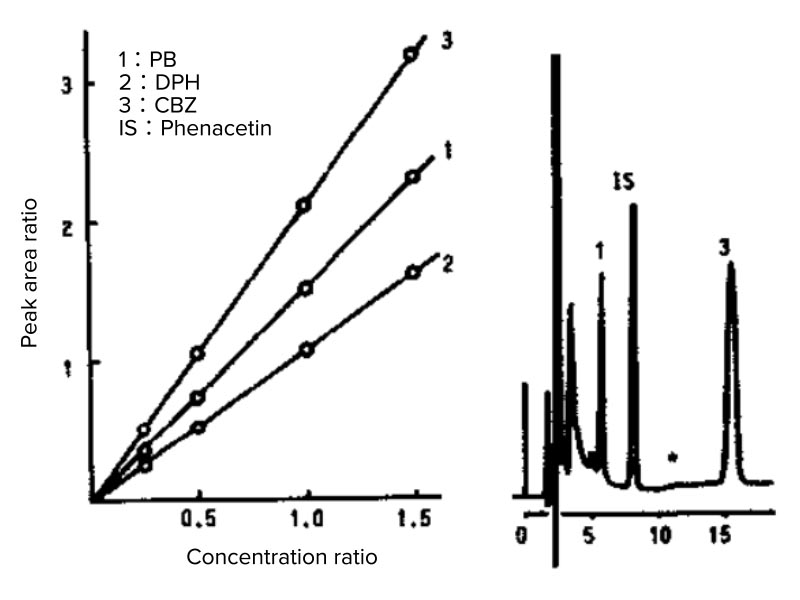
There are two methods for quantification: the external and internal standard methods, both of which are performed using a calibration curve. The external standard method creates a calibration curve for a standard sample and unknown samples are quantified using the calibration. In the internal standard method, a fixed amount of an internal standard substance is added to an unknown sample when creating a calibration curve using a standard sample, and a calibration curve is created with the concentration ratio vs. peak area ratio for quantification. As shown in Figure 6, the internal standard substance is required to be a component not included in the actual sample, to produce peaks that are completely separable from those for any contamination components, to elute at a retention time close to the quantitative target component, to be chemically and physically stable, and to be highly pure.
The advantage of the internal standard method is that it prevents errors in the injection volume or those caused by evaporation of the solvent.
©Jasco Europe S.R.L. (Socio Unico) Direzione e coordinamento ex. art. 2497 bis c.c. – P.I. 08609570158 | Privacy Policy | Cookie Policy | Manage consent | Realizzazione sito web: Alkimedia
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| CookieLawInfoConsent | 1 year | Records the default button state of the corresponding category & the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | This cookie is used by the website's WordPress theme. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
| Cookie | Duration | Description |
|---|---|---|
| Google Maps | Google Maps is a map visualization service managed by Google Inc. and is used to integrate such contents within its pages. |